Statement Problem: Jake and Joshua have new jobs selling gift cards at a local convenience store at the cash register, but their pay is different. Jake earns a foundational wage of $15 per hour, as well as $3 for each gift card sold. Joshua gets $4 for each gift card sold and earns a foundational wage of $10 per hour. If they each sell a certain number of gift cards in one hour, they will end up earning the same amount of pay. How many gift cards would that make up to?
Solution:
Let the certain number of gift cards they each sell in one hour be g;
Thus, for that hour, Jake earns;
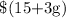
and Joshua earns;
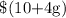
If they end up earning the same amount of pay for that hour, then;
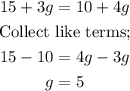
Thus, they each sell five giftcards.