ANSWER
The pressure of the gas is 8.21 atm
EXPLANATION;
Given that;
The volume of the gas is 816 liters
The temperature of the gas is 786 K
The number of moles of the gas is 104 moles
Follow the steps below to find the pressure of the gas
Step 1; Assume the gas behaves like an ideal gas, then, the pressure of the gas can be calculated using the ideal gas equation
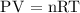
Step 2; Substitute the given data into the formula above

Hence, the pressure of the gas is 8.21 atm