Answer: (15.56mL +15.52mL)/2=15.54mL
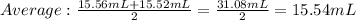
Explanation: The volume reading is called a titer value. For precision the values should be within 0.05mL of each other. Values that do not fall within this region is called an outlier is not used to find the average titer value.