The formula for the density, ρ, is (given a mass m and a volume V):
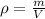
We know the volume in the graduated cylinder before and after the addition of the metal cylinder, so the difference is the volume of the metal cylinder:
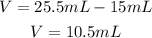
And the mass is given:
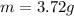
So, the density is:
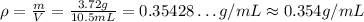
The density of the metal cylinder is approximately 0.354 g/mL.