Answer:
6.67moles
Explanations:
Given the balanced chemical reaction between hydrogen and oxygen expressed as:
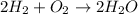
First, you need to calculate the moles of water produced.
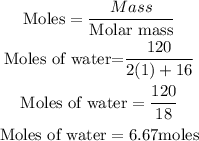
Based on stochiometry, 2 moles of hydrogen produces 2 moles of water, hence the moles of hydrogen that will be needed to produce 120g of water is 6.67moles