Given:
The mass of the ice is m = 150 g
Required: The heat required to convert ice to water at zero degrees Celsius.
Step-by-step explanation:
The heat can be calculated by the formula
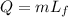
Here, the latent heat of fusion is
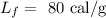
On substituting the values, the heat will be
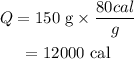
Final Answer: The heat required to convert ice to water at zero degrees Celsius is 12000 calories.