Step 1 - Discovering the ionic formula of Ammonium Oxide
Ammonium Oxide is formed by the ionic bonding between Ammonium (NH4(+)) and Oxide (O(2-)):
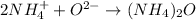
Step 2 - Finding its molar mass
To find the molar mass of a substance, we need to multiply the molar mass of each element by the number of times it appears in the formula and, finally, sum it all up. The molar masses are 14 g/mol for N; 1 g/mol for H and 16 g/mol for O. We have thus:
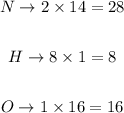
The molar mass of (NH4)2O will be thus:
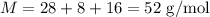
Step 3 - Finding the percent composition of Oxygen
As we saw in step 2, the molar mass of ammonium oxide is 52 g/mol. From this mass, 16 g/mol come from Oxygen. We can calculate the percent composition of Oxygen by setting the following proportion:
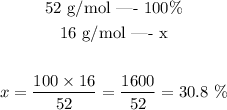
The percent composition of Oxygen is thus 30.8 %.