Answer:
A loud popping noise. Option C is correct
Explanations:
The reaction between hydrogen and oxygen is as given below;
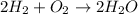
This reaction shows that hydrogen undergoes combustion (burning of hydrogen). This reaction cause an explosion of hydrogen which leads to a pop sound that hydrogen make during the reaction.
Hence the change that indicates a release of bond energy during the reaction is a loud popping noise.