1) List known and unknown values
Compound A: CO2
Molar Mass: 44.01 g/mol
Rate of effusion: 1.0
Compound B: CxH2n+2
Rate of effusion: 1.206
Molar mass:
2) Graham's law
![(Rate_A)/(Rate_B)=\sqrt[]{(MM_B)/(MM_A)}](https://img.qammunity.org/2023/formulas/chemistry/college/4089y1lxne0etfnk2ax13ajsmz4ieeqv7o.png)
Plug in values and solve for MMB
![(1.0)/(1.206)=\sqrt[]{\frac{MM_B}{44.01\frac{g}{mol_{}}}}](https://img.qammunity.org/2023/formulas/chemistry/college/lkibfls6rfv7if1gf25ctkr2pzxvavuy05.png)
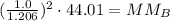
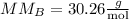
The molar mass of CxH2n+2 is 30.26 g/mol
3) The molecular mass of the unknown compound
The molar mass of CH4 is 16.04 g/mol
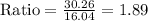
The ratio is 2. So, x is equal to 2
.