21.976g of glucose are produced from 30.0g ofcarbon dioxide.
In the equation of the photosynthesis process, carbon dioxide (CO2) reacts with water (H2O) to produce glucose (C6H12O6) and oxygen (O2):
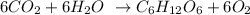
1st) It is necessary to calculate the amount of carbon dioxide and glucose with the relation of moles in the photosynthesis equation. We can calculate that with the molar mass of carbon dioxide and the molar mass of glucose:
- Carbon dioxide molar mass: 44g/mol
Grams of CO2 in the equation:
6mol x 44g/mol = 246 g of CO2
- Glucose molar mass: 180.2g/mol
Grams of C6H12O6 in the equation:
1mol x 180.2g/mol = 180.2 g of C6H12O6
Now we know that 246g of CO2 are consumed to produce 180.2g of C6H12O6.
2nd) With the calculated values we can use a mathematical Rule of Three to calculate the grams of glucose that are produced from 30.0 of carbon dioxide:
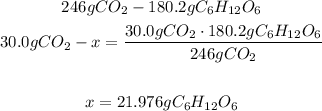
So, 21.976g of glucose are produced from 30.0g ofcarbon dioxide.