Answer
14.7 grams of Fe
Step-by-step explanation
Given:
Mass of CO = 14.2 g
Mass of Fe₂O₃ = 21.0 g
What to find:
The grams of iron metal (Fe) produced.
Step-by-step solution;
The first step is to write a balanced chemical equation for the reaction:
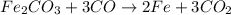
The second step is to determine the limiting reactant
From the Periodic Table:
1 mol CO = 28.01 g
1 mol Fe₂O₃ = 159.69 g
1 mol Fe = 55.85 g
From the balanced equation;
3 mol x 28.01 g/mol = 84.03 g CO reacts with 1 mol x 159.69 g/mol = 159.69 g Fe₂O₃
So 14.2 g CO will react with
![\frac{14.2\text{ g CO}*159.69\text{ g Fe^^^^2082O^^^^2083 }}{84.03\text{ g CO}}=27.0\text{ g Fe^^^^2082O^^^^2083}]()
This implies that the given 14.2 g of CO will require 27.0 g Fe₂O₃. Note that the given mass of Fe₂O₃ that reacted is 21.0 g, hence Fe₂O₃ is the limiting reactant.
The final step is to use the limiting reactant to calculate the grams of Fe metal produced.
If from the balanced equation, 159.96 g Fe₂O₃ produced 2 x 55.85 g = 111.7 g Fe,
Therefore, 21.0 g Fe₂O₃ will produce:
![\frac{21.0\text{ g Fe^^^^2082O^^^^2083}*111.7\text{ g Fe}}{159.96\text{ g Fe^^^^2082O^^^^2083}}=14.7\text{ grams of Fe}]()
Hence, 14.7 grams of iron metal can be produced from the reaction of 14.2 g of CO gas with 21.0 g of Fe₂O₃