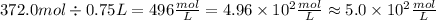There are some rules for significant figures in operations.
If we are multiplying or dividing two numbers, the amount of significant figures in the restul has to be the same as the number with lowest significant figures.
So, in the first case, we have:
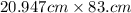
As we can see, the fist number has 5 significant figures, while the second has only 2. This means that the result has to be 2 significant figures.
So, we first calculate it normally:
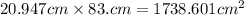
Now, we write in scientific notation:
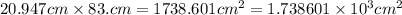
Now, we approximate the number so it has only 2 significant figures. We can see that we will have to approximate so it has 1 decimal place, that way we will have two significant figures:
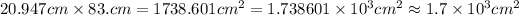
Now, we repeat for the others.
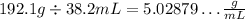
We can see that the first have 4 significant figures and the second has 3, so the result has to have only 3:
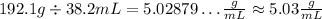
For the last one, we have:
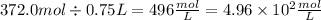
Now, we can see that the first number has 4 significant figures, because decimal trailing zeros count as significant figures. The second has only 2 significant figures, because leading zeros don't count as significant figures. Thus the resulta has to have only 2 significant figures: