The mass percent is described by the following equation:
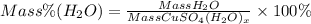
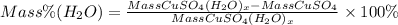
CuSO4 corresponds to anhydrous copper (II) sulfate
CuSO4(H2O)x will be hydrate of copper sulfate
H2O corresponds to the water. The mass of water as we saw in the equation is the total mass of hydrate of copper sulfate minus the mass of anhydrous copper (II) sulfate
So, we can replace the known masses:
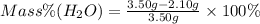
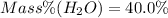
Answer: The mass percent of water in the hydrate is 40.0%