Answer:
6.0 moles.
Step-by-step explanation:
To solve this problem, we have to use the molarity formula. Remember that the molarity (M) of a solution is the number of moles of solute dissolved in one liter of solution:
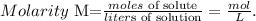
We want to find 'moles of solute', so we have to solve for this unknown value and replace the given data (molarity = 2.0 M, liters of solution = 3.0 L):
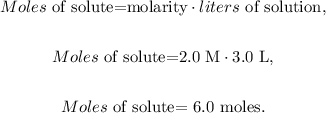
The answer would be that we require 6.0 moles to prepare the wanted solution.