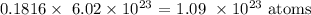Answer:
In 7.1g of potassium, there are 1.09 * 10^23 atoms
Step-by-step explanation:
HERE, we want to get the number of atoms on 7.1g of potassium
The first thing we have to do here is to convert the given mass to moles
We can do this by dividing the mass by the atomic mass unit of potassium
The atomic mass unit of potassium is 39.1 amu
We can get the number of moles mathematically as follows:
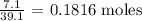
Finally,we can get the number of atoms
In 1 mole of a substance, the number of atoms is:
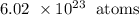
To get the number of atoms in 0.1816 mole, we simply multiply the two as follows: