Let's see that the formula to find the volume using density and mass is:
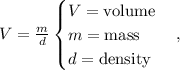
In this case, the problem is asking about the volume in mL but remember that mL is the same that cm^3, so using the formula we're going to obtain:
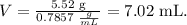
The volume of 5.52 g of acetone is 7.02 mL.