ANSWER
The new volume of the gas is 162.28L
Step-by-step explanation
Given that;
The initial temperature of the gas is 202K
The initial pressure of the gas is 1520 torr
The initial volume of the gas is 60.0L
The final temperature of the gas at STP is 273.15K
The final pressure of the gas at STP is 760 torr
Follow the steps below to find the final volume of the gas
Step 1; Write the general gas law equation
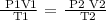
Step 2; Substitute the given data into the equation above to find the final volume of the gas

Therefore, the new volume of the gas is 162.28L