So, using the formula:
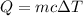
We could replace the values of the question:
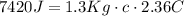
Given that "c" is the specific heat of the substance, we could find it solving the previous equation:
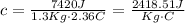
If we look, this specific heat corresponds likely to Glycerine. That's because glycerine has a specific heat equals 2410J/KgC , and this value is extremely similar to the value that we found.