We have 2.054 grams of nickel (Ni) and 2.482 grams of chlorine (Cl2). The total mass of the compound is 4.536 g (This results by the sum of the masses: 2.054 g + 2.482 g).
Let's find the composition of nickel and chlorine, using the following formula:
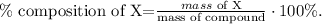
X would be Ni and Cl2:
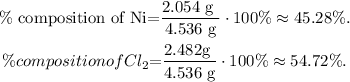
The answer would be 45.28 % of Ni and 54.72% of Cl.