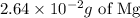Step 1 - Understanding the gas equation
The volume (V), the pressure (p) and the temperature (T) of a gas samples can be related to its number of moles (n) by the gas equation:
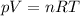
In this equation, R represents the universal gas constant, which may have different values depending on which unities are used.
Let's use L for volume, mmHGg for pressure and K for temperature. In these conditions, R = 62.3 L.mmHg/K.mol.
Step 2 - Calculating how many moles of H2 gas are produced
Now let's set the values in the equation. The exercise states that:
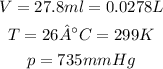
Substituting in the equation:
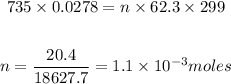
Therefore, 1.1*10^(-3) moles of H2 gas were produced.
Step 3 - Calculating the required mass of Mg
The given equation is:
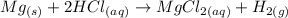
We can see that one mole of Mg produces one mole of H2 gas. This is a fixed proportion. We will always have the same amount of Mg and H2 in moles for this reaction.
Therefore, if 1.1*10^(-3) moles of H2 were produced, it means the same amount of moles of Mg were required: 1.1*10^(-3) moles.
We can convert this value to mass of Mg by multiplying it by its molar mass (24 g/mol)
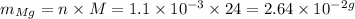
We would need thus 2.64*10^(-2) g of Mg for this reaction.
Answer: