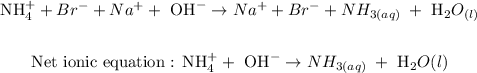ANSWER
Step-by-step explanation
Given compound
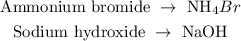
The next step is to write the molecular equation of the reaction between ammonium bromide and sodium hydroxide
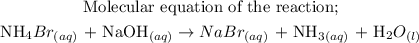
The next step is to write the total ionic equation.
To write the total ionic equation, follow the steps below
1. Break the compounds that exist in the aqueous state into ions
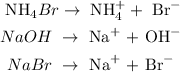
2. Combine the ions together
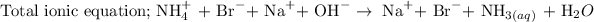
The last step is to write the net ionic equation
To write the net ionic equation, you have to cancel ions that appear on the reactants sides, and the products side