Explanation:
What to find? The volume of perchloric acid
Firstly, we need to write the balanced equation of the reaction
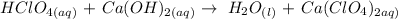
Given the following parameters
The concentration perchloric acid = 0.224M
The concentration of Calcium hydroxide = 0.149 M
The volume of Calcium hydroxide = 26.0mL
The volume of perchloric acid =?
Recall that the equivalentce point of a titration is
mole acid = mole base
Mole = Molarity x volume