Answer:
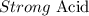
Step-by-step explanation:
Here, we want to classify H2SO4
A strong acid is one that will completely dissolve in an aqueous solution while a weak acid is one that does not completely dissolve in an aqueous solution
Sulfuric acid completely dissolves in an aqueous solution and as such is a strong acid