The first step we have to follow to answer this question is to calculate the new pressures of each of the containers.
Since pressure in Container A is tripled, now it will be:
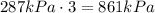
Since pressure in Container B is reduced by one third, now it will be:
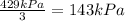
Now, according to Dalton's Law the total pressure of a mixture of gases is the sum of the pressures of each component in the mixture. To find the total pressure in Container C we have to find the sum of the new pressures:
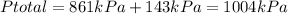
It means that the pressure in Container C is 1004kPa.