Step 1 - Understanding the reaction
The given reaction is a precipitation reaction. In order to discover the spectator ions, we need to discover which substance precipitates, i.e., which one is insoluble in water.
The two products are PbSO4 (insoluble in water) and HNO3 (soluble in water). Therefore, only the ions Pb2+ and SO4(2-) are effectively reacting, while the remaining ions, H+ and NO3-, are spectator ions.
We can rewrite this equation in ionic formula:
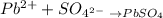
Step 2 - Answering the exercise
The spectator ions are thus:
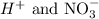
Answer: H+ and NO3-