So,
First remember that the work done can be found if we remember the following equation:
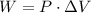
Where W is the work done, P is the pressure (constant) and ΔV represents the change in the volume. (The substraction between the final volume and the initial volume)
If we replace our values, we got that:
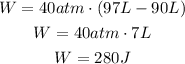
The gas is being expanded, the sign of the work done is positive.
The work done is 280J.