1) Define the equation for calculating mass percent.
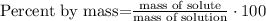
2) The mass percent of chloroform
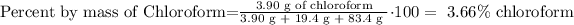
The mass percent of chloroform is 3.66% chloroform.
3) The mass percent of acetyl bromide
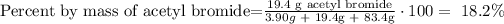
The mass percent of acetyl bromide is 18.2% acetyl bromide
4) The mass percent of heptane
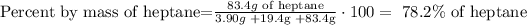
The mass percent of heptane is 78.2% of heptane