Answer
144.564 grams
Exlanation
The given moles of MgO = 3.500 moles
The mass in grams of MgO can be calculated using the mole formula.
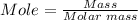
From the periodic table, the atomic masses of (O = 15.999, Mg = 24.305)
Molar mass of MgO = (24.305 + 15.999) = 40.304 g/mol
Putting mole = 3.500 mol and molar mass = 40.304 g/mol into the mole formulag/mol
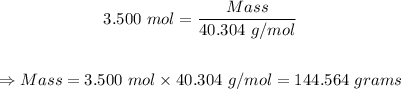
Therefore, the grams of magnesium oxide (MgO) that are in 3.500 moles of magnesium oxide = P144.564 grams