Answer:
1 mole KClO3 / 122.55 g KClO3,
3 mole O2 / 2 mole KClO3,
31.998 g O2 / 1 mole O2.
Step-by-step explanation:
First, let's convert 10 g of KClO3 to moles using the molar mass of KClO3 which is 122.5 g/mol:
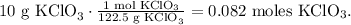
Now you can see in the chemical equation that 2 moles of KClO3 reacted produces 3 moles of O2, so we state a rule of three to find the number of moles of O2:
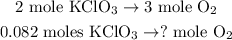
The calculation of this will look like this:
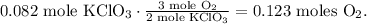
The final step to finding the mass of oxygen (O2) is using its molar mass which is 32 g/mol, like this:
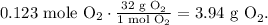
As you can see from this process, the needed ratios are:
1 mole KClO3 / 122.55 g KClO3,
3 mole O2 / 2 mole KClO3,
31.998 g O2 / 1 mole O2. (in the explanation is approximated)