Here, we want to write and solve an exponential equation.
The exponential equation can be written as;
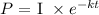
Where P represents the present mass to be calculated
I is the initial mass given
k is the radioactive decay constant
and t is the time taken to reach the present given mass.
From the question;
P = 25 grams
I = 500 grams
t = ?
k can be calculated using the equation;
k = ln 2/half-life
From the question, the half-life is given as 15 hours
Hence, l = ln 2/15
Substituting this in the decay equation, we have;
25 = 500 * e^-(ln 2/15 * t)
divide both sides by 500
0.05 = e^-(0.0462 * t)
0.05 = e^(-0.0462t)
Find the ln of both sides
ln 0.05 = ln e^(-0.0462t)
-2.996 = -0.0462t
t = 2.996/0.0462
t = 64.848
Rounding this to the nearest tenth is 64.8 years
Hence, it will take the isotope 64.8 years to decay to 25 grams