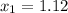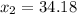Answer: 1.12 ≤ x ≤ 34.18
Step-by-step explanation
As the company wants to keep its profits at or above $75,000, we have to search for those values of tires produce (x) that maintain the profits at or above $75,000. Based on this we can conclude that the negative numbers do not give this profit, and 0 tires is below $75,000. Thus, the best approximation is:
Additionally, we can prove this by making the function equal 75:
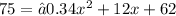
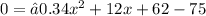
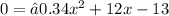
Using the General Quadratic Formula we get that: