Step 1 - Finding the formula of Iron (III) Oxide
Iron (III) Oxide is the resulting compound when two ions, Iron (III), Fe(3+), and O(2-) combine with each other. The resulting compound formula will be thus:
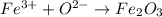
Step 2 - Understanding what "constituent elements" means
When we refer to constituent elements we are talking about the most common form, found in nature, for the elements that compose a substance. Water, for example, is composed of the elements H and O (H2O).
The most common form of H atoms in nature is H2, hydrogen gas. The same is valid for O atoms (O2 gas). Therefore, the equation for forming water from its "constituent elements" would be:
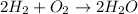
Therefore, for the substance Fe2O3, the elements that compose it are Fe and O. O, as we saw, is found in the form of O2. But what about Fe? Well, Fe can be found as pure metal, which is represented as Fe only.
The equation for forming Fe2O3 from its "constituent elements" is thus:
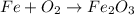
Step 3 - Balancing the equation
To properly balance a chemical equation, we must guarantee the number of atoms of each element is the same in both sides of the equation.
Let's count the number of atoms in the previous reaction:
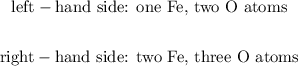
As we can see, this reaction is not balanced. We can start balacing it by multiplying Fe by 2:
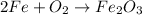
The quantity of Fe atoms is now properly balanced. Now, to balance O2, we can multiply it by 3/2:
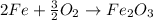
If you feel unconfortable workin with fractions, there's no problem at all. Just multiply the whole equation by the denominator:
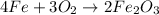
The correct alternative is thus item a.