Answer
2.24 L
Step-by-step explanation
Given:
volume of nitrogen dioxide produced = 4.50 L
The given unbalanced equation is;
___ NO (g) + ___O2 (g) → ___NO2 (g)
What to find:
The liters of gaseous oxygen needed to produce 4.50 L of gaseous nitrogen dioxide at STP.
Step-by-step solution:
The first step is to write a balanced equation for the reaction.
2 NO (g) + 1 O2 (g) → 2 NO2 (g)
The next step is to convert 4.5 L NO2 to moles.
Conversion factor: 1 mole of any gas at STP occupies 22.4L.
Therefore, the number of moles of NO2 in 4.5 L volume will be
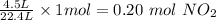
The next step is to calculate the mole of O2 needed to produce 0.20 mol NO2.
From the balanced equation 1 mol O2 produce 2 mol NO2
So, the x mol O2 will be needed to prduce 0.20 mol NO2
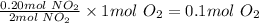
The final step is to convert 0.1 mol O2 to liters using the conversion factor in step 2.
The liters of gaseous oxygen needed is
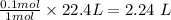
The liters of gaseous oxygen needed to produce 4.50 L of gaseous nitrogen dioxide at STP = 2.24 L