Answer:
2.651 * 10⁴kg
Explanations:
The balanced reaction between limestone (CaCO₃) and sulfuric acid (H₂SO₄) is given as:
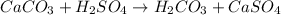
Given the following parameters
• volume of sulfuric acid = 5.2 x 10⁹ L
,
• Mas of sulfuric acid = 0.005g
Determine the moles of sulfuric acid.
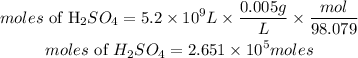
According to stochiometric, 1 mole of limestone reacted with 1mole of sulfuric acid, hence the number of moles of limestone required is 2.651 * 10⁵moles
Determine the required mass of limestone
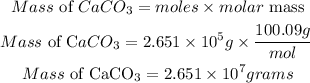
Convert the result to kilogram
Recall that 1000g =1kg
Hence;
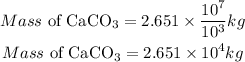
Hence the mass of limestone in kg would be required to completely neutralize a 5.2 x 10⁹ L lake containing 5.0 x 10-3 g of H₂SO₄ per liter is 2.651 * 10⁴kg