there is not value that satisfies the inequality
no solution
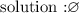
Step-by-step explanation
An absolute value function is a function that contains an algebraic expression within absolute value symbols. Recall that the absolute value of a number is its distance from 0 on the number line
it is given by
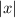
as, it is a distance the value is always positive ( or greater than zero)
Step 1
hence if we have the expression
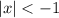
we are searching for all the values whose absolute value is smaller than -1
as we saw in the explanation, the absolute values are positive ALWAYS , hence,
there is not value that satisfies the inequality
in other words,
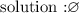
I hope this helps you