To calculate this, we can use fractions for equivalent amounts.
So, we have the number of atoms, using the Avogadro's Constant, we know that 1 mol of Mg is approximately 6.02 x 10^23, so:
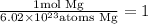
And since we have the number of atoms of Mg, we can convert to mol of Mg by multiplying by this fraction.
Now, from mol of Mg we can convert to mass using the molar mass of 24.30 g/mol. It says that 24.30 g og Mg is equivalent to 1 mol, so:
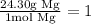
So, we have:
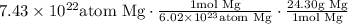
Notice that this is presented in a table in the question, so we can now just multiply all of them:
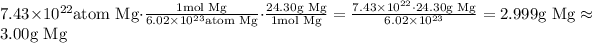
Thus, this is equivalent of approximately 3.00 g of Mg.