Step 1
The equations used here:
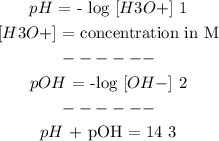
------------
Step 2
Information provided:
[H3O+] = 3.5×10^−7 M
------------
Step 3
Procedure:
Firstly, from (1):
pH = -log [H3O+]
pH = -log (3.5×10^−7 M) = 6.5
----
From (3):
pH + pOH = 14
pOH = 14 - pH
pOH = 14 - 6.5
pOH = 7.5
----
From (2)
pOH = - log [OH-] => [OH-] = 10^-(pOH)
[OH-] = 10^-(pOH) = 10^-(7.5) = 3.2x10^-8 M
Answer: [OH-] = 3.2x10^-8 M