ANSWER
The mass of glucose in grams is 230 grams
Step-by-step explanation
Given information
The total volume of the solution = 1.5L
Follow the steps below to find the mass of glucose
Step 1: Convert the volume of the solution from L to mL
According to the standard conversion, 1L is equivalent to 1000mL
Let x represents the volume of the solution in mL
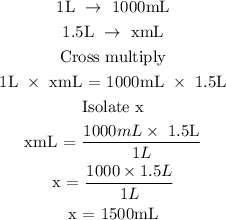
Hence, the volume of the solution in mL is 1500mL
Step 2: Find the mass of the glucose in grams
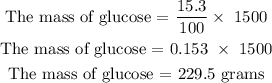
Hence, the mass of glucose in grams is 229.5 grams