Answer:
0.3405moles.
Explanations:
In order to get the moles of sugar, we need to get the final volume using the dilution factor as shown below:
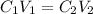
where:
• C1 and C2 are the, initial and final ,concentration
,
• V1 and V2 are the, initial and final, volume
Given the following parameters:
C1 = 2.27M
V1 = 150mL = 0.15L
C2 = 1.88M
V2 = ?
Substitute the given parameters into the formula:
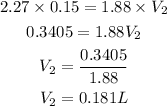
Next is to get the moles of sugar in the lemonade solution using the formula below:
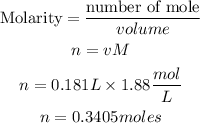
Hence the number of moles of sugar that are in the lemonade solution is 0.3405moles.