ANSWER
The pH of the solution is 12.75
EXPLANATION;
Given that;
The volume of the solution is 2500mL
The mass of calcium hydroxide is 10.4g
Follow the steps below to find pH of the solution
Step 1; Convert the volume to L
Recall, that 1mL is equivalent to 0.001L
Let the volume of the solution be x
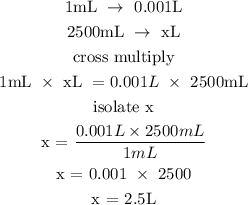
Step 2; Find the mole of calcium hydroxide using the formula below
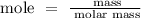
Recall, that the molar mass of calcium hydroxide is 74.093 g/mol

Step 3; Find the molarity of calcium hydroxide

Since calcium hydroxide is a base, then calculate the pOH of calcium hydroxide
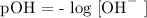
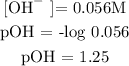
Step 5; Find the pH of the solution
Recall, that pOH + pH = 14
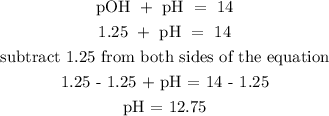
Therefore, the pH of the solution is 12.75