The formula relating mass, density, and volume is:
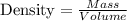
Let's find density of both of them.
1. Helium
Mass = 12.99
Volume = 29 ml
So, Density is:
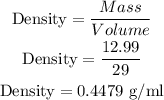
2.Carbon Dioxide
Mass = 17.2
Volume = 75 ml
So, Density is:
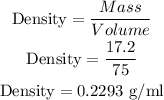
Of course, the denser gas would be helium.
Last Answer Choice is right, Helim with 0.44 g/ml