Given the following reaction:
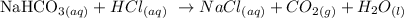
We want to know how many grams of NaHCO3 will react with 169 mL (0.169 L) HCl with 0.07640 M concentration.
Step 1: Find the moles of HCl
C = n/V
n = CV
n = 0.07640 M x 0.169 L
n = 0.0129 mol
Step 2: Find the moles of NaHCO3 then convert them to mass.
number of moles of NaHCO3 will be determined using the stoichiometry.
The molar ratio between NaHCO3 and HCl is 1:1
Therefore the number of moles of NaHCO3 = 0.0129 mol.
Now we can find the mass:
n = m/M
m = n x M
m = 0.0129 x 84,007 g/mol
m = 1.085 g