Answer
3 mol N2
Step-by-step explanation
Given:
Moles of H2 = 9.0 mol
Equation: N2(g) + 3H2(g) → 2NH3(g).
What to find:
The moles of nitrogen gas required to completely convert 9.0 mol H2 gas to NH3 gas.
Solution:
From the Balanced equation of reaction given;
3 mol H2 completely reacts with 1 mol N2
∴ 9.0 mol H2 will require
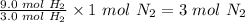
Hence, the moles of nitrogen gas required to completely convert 9.0 mol H2 gas to NH3 gas is 3 mol N2.