Answer:
The products of the reaction are aluminum sulfate (aqueous) and copper (solid).
Step-by-step explanation:
The balanced equation is:
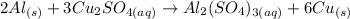
The equation is balanced because there 2 Al, 6 Cu, 3 S and 12 O in each side of the reaction.
Sulfate ion is the negative part of copper (I) sulfate, so it will react with aluminum to form aluminum sulfate, and the products will be aluminum sulfate (aqueous) and copper (solid).