Given data
The initial volume of the ballon is v = 2 L
The initial temperature is T = 20 C = 293 K
The ballon is placed inside the refrigerator at a temperator of T_f = - 18 C = 255 K
The pressure remains constant, the expression for the volume temperature relation is given as:
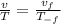
Substitute the value in the above equation.
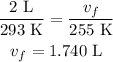
Thus, the final size of the ballon is 1.740 L.