They tell us that it is a sealed glass, which means that there is no entry or exit of matter, assuming that the temperature remains constant we can say that the pressure is also constant.
Now, when we have a mixture of gases, the sum of their partial pressures will be equal to the total pressure, therefore we have the following equation:
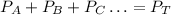
Where PA, PB and PC refer to the partial pressures of gases A, B, and C.
PT is the total pressure.
We have in the question three gases: Carbon Monoxide (CO), Carbon dioxide (CO2), and oxygen (O). The total pressure is 2.68 atm. We can replace the know values into the equation and clear the Partial pressure of carbon.
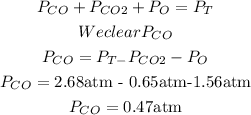
The partial pressure of carbon monoxide is 0.47 atm