• Given:
Work done = 1.85 x 10⁸ J
Efficiency = 4%
Let's solve for the following:
• (a). How much heat transfer (in J) occurs to the environment?
Apply the formula:
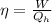
Where Qh is the amount of heat transfer.
rewrite the formula for Qh:
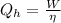
The equation for the work done by the engine will be:
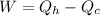
Now, substitute W/n for Wh in the equation for the work done:
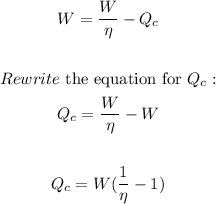
Where:
n is the efficiency = 4% = 0.04
W is the work done.
Plug in the values and solve for Qc:
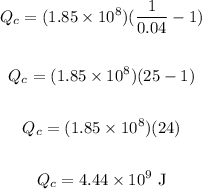
Therefore, the amount of heat transfer that occurs in the environment is 4.44 x 10⁹ J.
• Part B:
Given:
Amount of heat produced by each barrel when burned = 6.00 x 10⁹ J.
To find the amount of barrels of fuel consumed, apply the formula:
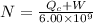
Substitute values in the formula and solve for N, where N is the number of barrels of fuel burned.
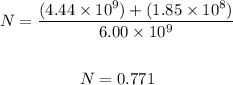
Therefore, the amount of fuel consumed is 0.771 barrels.
ANSWER:
• (a). 4.44 x 10⁹ J
• (b). 0.771 barrels.