We need to calculate the work done and the increase in external energy. The external work can be calculated as:
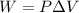
where P is the pressure and delta V denotes the change in volume.
The increase en external energy is given by:
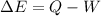
where W is the work done by external forces and Q is the heat transfer.
Before we begin we convert the units to the correct units:
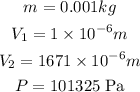
a)
Plugging the values we know we have that:
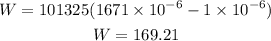
Therefore, the work done is 169.21 J
b)
The heat transfer is given by:
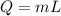
then we have:
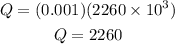
Once we know this we have that the change in external energy is:
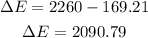
Therefore, the increase in external energy is 2090.79 J