The percent composition of a compound is its mass percent with respect to the sample total mass.
We already know tha sample mass, 200.0 g, to find the mass of the carbon dioxide, CO₂, we can use its molar mass and the measured number of moles:
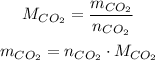
We know that the molar mass is 44.01 g/mol, and the number of moles is 0.30 mol, so:
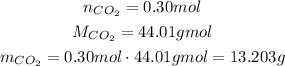
Now, we check the percent of 13.203g with respect to the total 200.0 g:
200.0g ---- 100%
13.203g ---- P
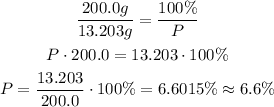
By comparison, we can see that this corresponds to alternative D.