Step 1
Molarity is defined as follows:
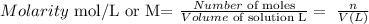
---------
Step 2
Data provided:
V(L) = 2.85 L (solution)
Molarity = 0.10 M or mol/L
---------
Step 3
Procedure:
Molarity = n/V(L)
Molarity x V(L) = n
0.10 mol/L x 2.85 L = n
0.29 moles = n
Answer: 0.29 moles of NaCl